Histiocytic sarcoma complex - distinctive syndromes
There are several presentations of HS that deserve separate additional description because of particular clinical or clinic-pathological associations that are often crucial to successful diagnosis.
Hemophagocytic histiocytic sarcoma
Dogs with hemophagocytic HS present with a distinctive clinico-pathologic syndrome. These dogs usually have a moderate to marked, regenerative, hemolytic anemia and thrombocytopenia. They have mild hyperbilirubinemia without jaundice at initial presentation. Most dogs have hypoalbuminemia and often hypocholesterolemia. This clinical picture is often confused with immune-mediated hemolytic anemia and thrombocytopenia (Evans syndrome) despite the lack of erythrocyte bound IgG (Coombs’ negative) in tested dogs. Hemophagocytic HS carries the worst prognosis of all forms of HS with a median survival of only 4 weeks (mean 7 weeks) after diagnosis. Factors contributing to poor outcome include development of severe anemia and coagulopathy with disease progression (Vet Pathol. 2006;43(5):632–645.). In another study, in which the efficacy of CCNU was assessed as a treatment for HS, a cohort of dogs that presented with anemia, hypoalbuminemia and thrombocytopenia had a median survival of 28 days or less (versus 106 days for all dogs). The form of HS was not further classified in this subset of dogs, but the hematological and biochemical values were shared by hemophagocytic HS.
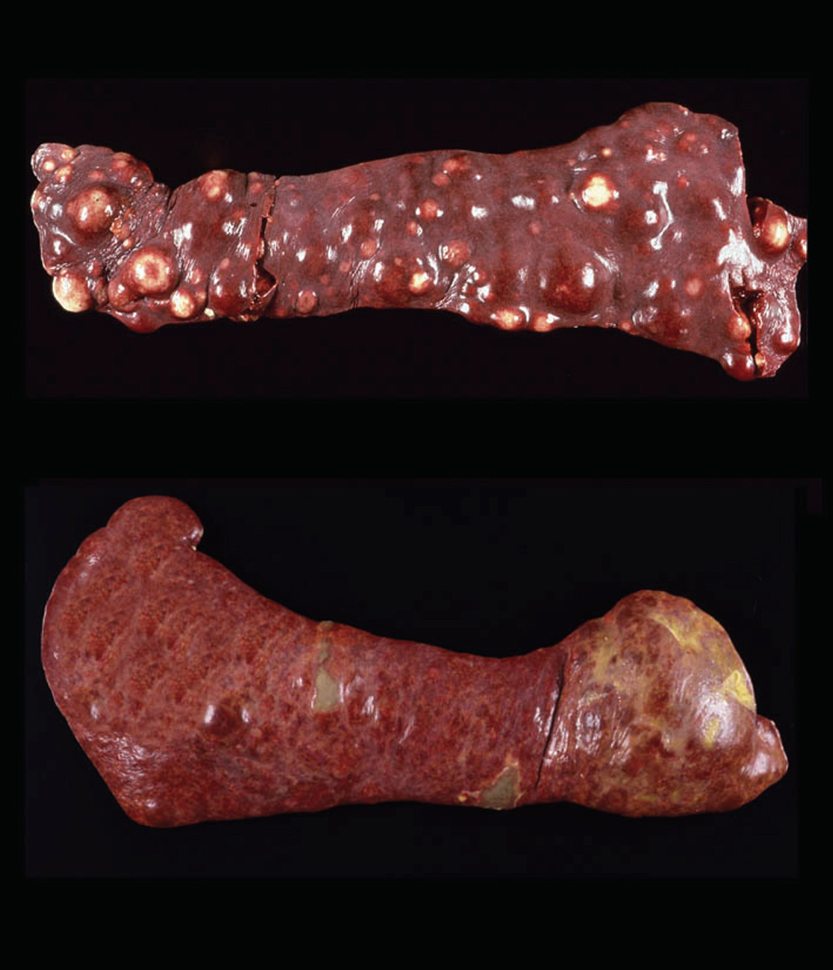
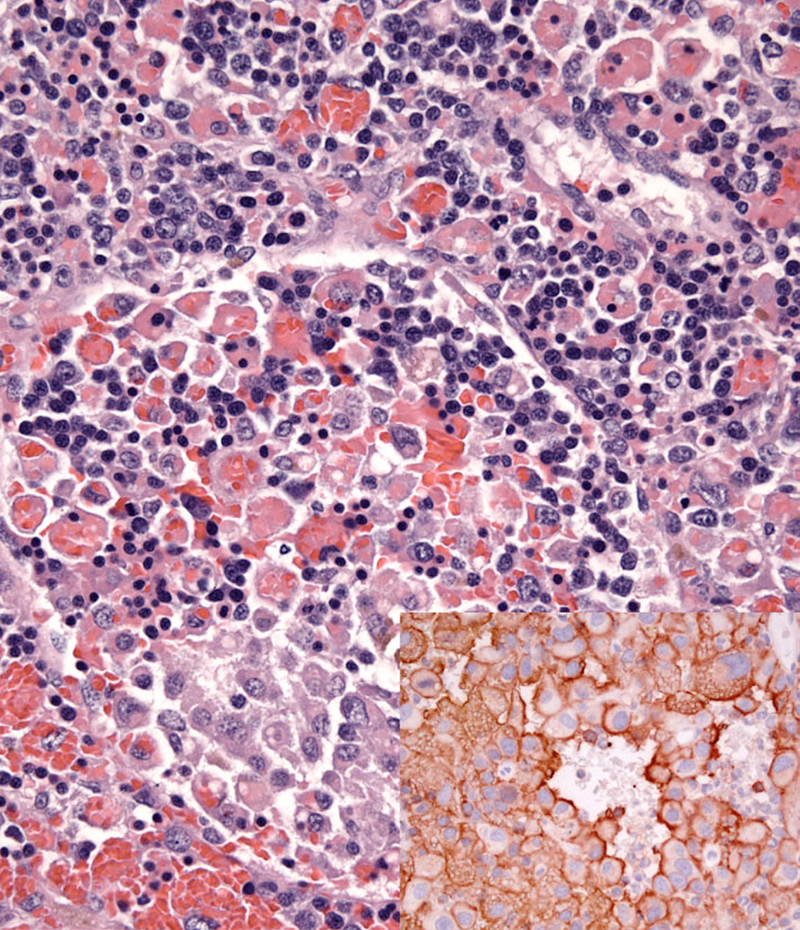
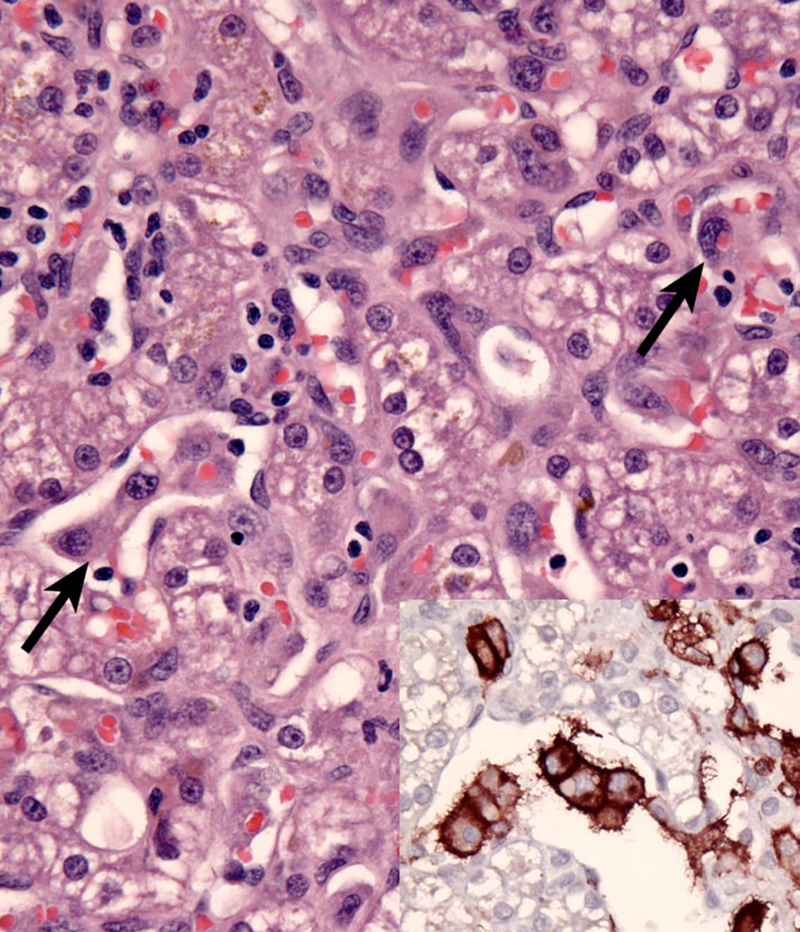
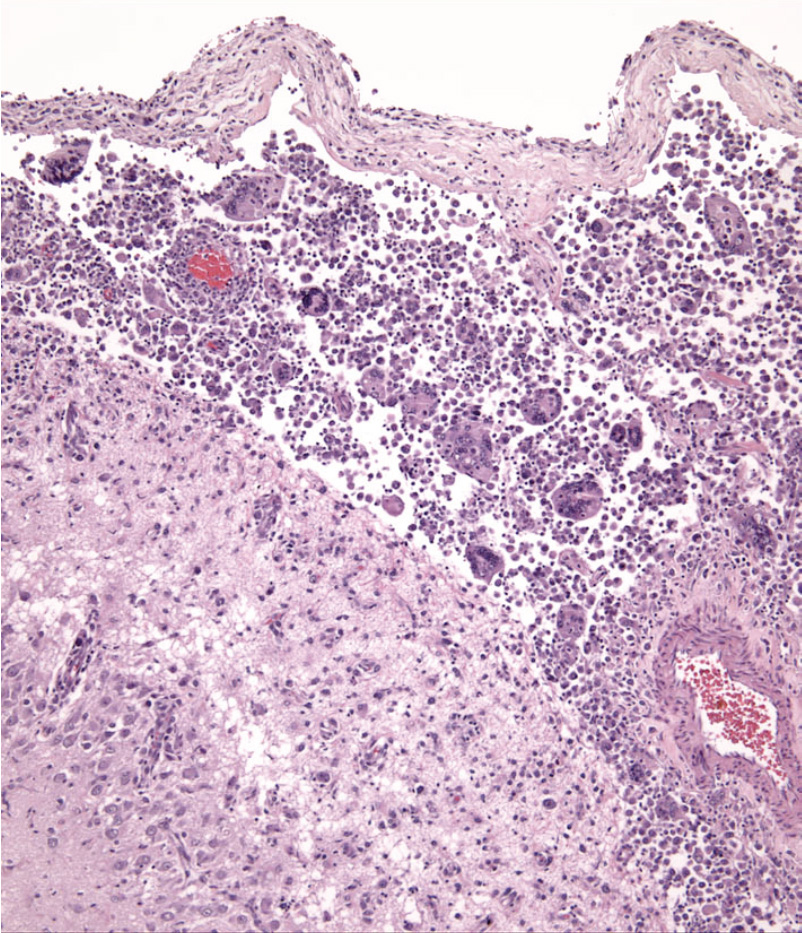
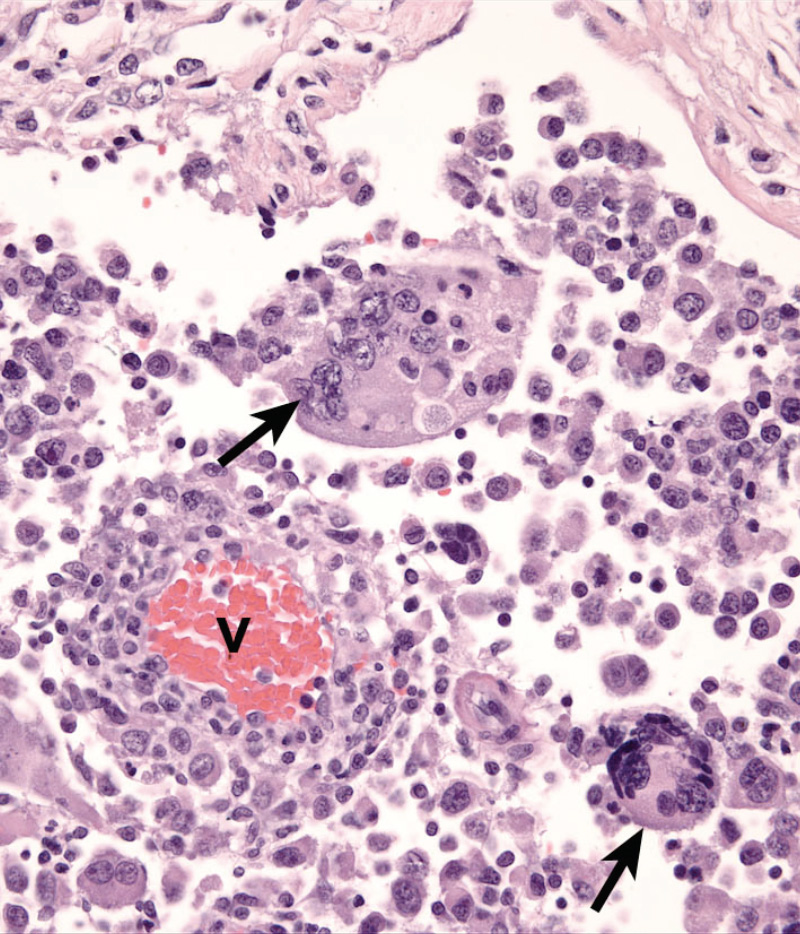
Dogs with hemophagocytic HS have diffuse splenomegaly as already described (Fig. 1), and they lack grossly visible masses (<1-2 mm) in metastatic sites such as liver and lung. This is enforced by the growth pattern of the neoplastic histiocytes. If diffuse splenomegaly and discrete masses are present, immunophenotypic investigation will reveal both types of histiocytic differentiation (DC and macrophage); this is an uncommon event. In hemophagocytic HS, histiocytes expand the splenic red pulp more or less diffusely. They exhibit marked erythrophagocytosis and are accompanied by interspersed foci of extra-medullary hematopoiesis (Fig. 2). They invade red pulp sinuses and travel to the liver where they insidiously invade the sinusoids (Fig. 3). Examination of sections of lung will reveal erythrophagocytic histiocytes within the pulmonary vasculature. The bone marrow is simultaneously infiltrated and erythrophagia is observed there as well. There are two reports of HS in cats, in which the lesions in three cats closely resembled the pattern and distribution of hemophagocytic HS of dogs.
Cytologically, the histiocytes in hemophagocytic HS may appear well differentiated (especially in bone marrow); in fact there is often asynchrony between spleen and bone marrow; usually there is more atypia in the spleen compared to bone marrow. However, even the well-differentiated cells are aggressive in their behavior, as evidenced by invasion of the liver and lung vasculature.
Neoplastic histiocytes in hemophagocytic HS express a distinctive surface antigen profile much like that expressed by macrophages in splenic red pulp and bone marrow. They express CD11d/CD18 (Fig. 2 inset) and lack expression of CD11c, and have variable CD1a expression – usually weak to negative. Both CD11d and CD18 are assessable in formalin fixed tissue sections, and this greatly facilitates the diagnosis of hemophagocytic HS, and reveals the often inconspicuous metastatic, intravascular lesions in liver and lung (Fig. 3 inset). Feline hemophagocytic HS is also thought to arise in macrophages, based on lack of CD1 expression. An origin from splenic red pulp macrophages could not be confirmed due to lack of expression of CD11d.
Articular / periarticular histiocytic sarcoma
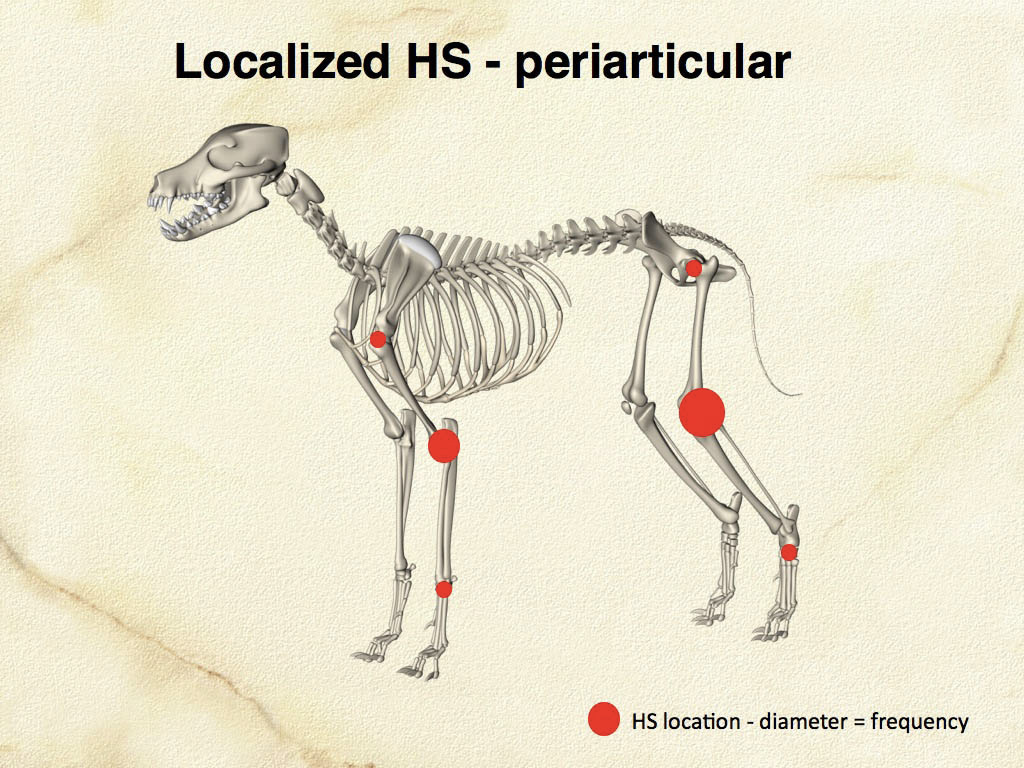
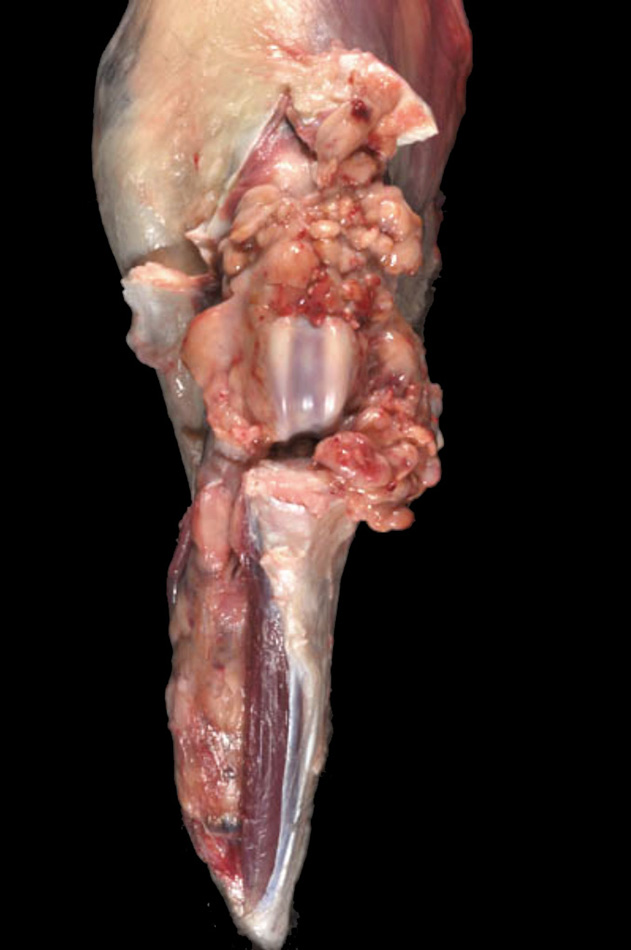
Articular HS is a localized form of HS, which occurs on the limbs. The lesions originate either within the joints (intra-articular) and/or adjacent to the joints (peri-articular) of the appendicular skeleton. Articular HS is the most common tumor affecting the joints of dogs, and also occurs in cats at much lower incidence. The stifle and elbow joint are most commonly affected, and lesions may occur in the coxofemoral joint and carpus (Fig. 4). More than half of the dogs were Rottweilers (Vet Pathol. 2002;39(1):66–73) Invasion of bone can occur with advanced disease. Since skeletal lesions are observed in disseminated HS in the same sites as articular HS, it is important to determine if the skeletal involvement is subsequent to a soft tissue mass originating near the synovial tissues (Vet Radiol Ultrasound.2007;48(6):539–543) Early detection and treatment of articular HS is potentially curative, while disseminated HS has a worse prognosis. However, articular HS is capable of widespread dissemination since histiocytes, especially those of the various DC lineages, are actively migratory cells. This is reflected in the median survival of dogs with articular HS and evidence of metastatic disease (about 5 – 8 months).
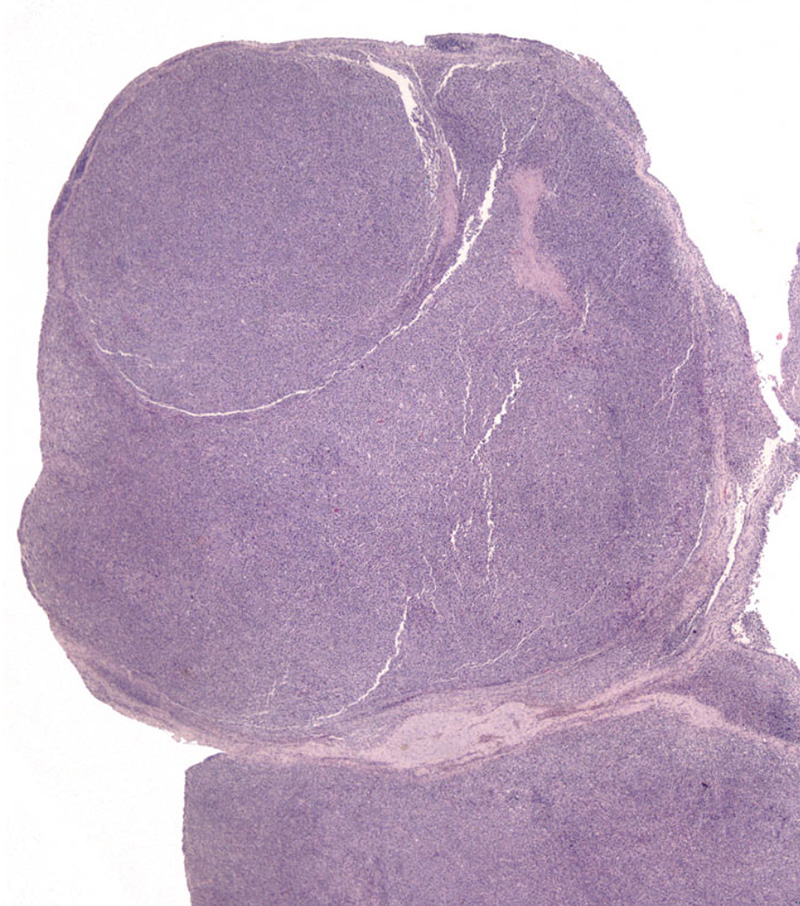
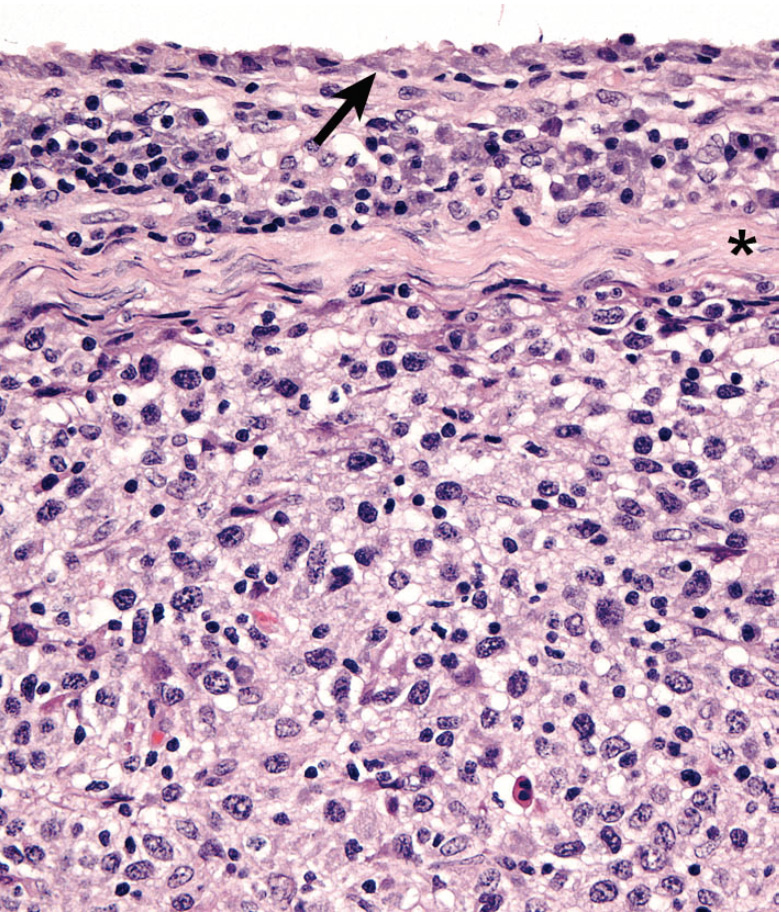
Articular HS has a distinctive appearance: it occurs as multiple tan nodules located beneath the synovial lining. Hence these lesions begin outside of the synovial space either within the joint and/or in a periarticular location. The lesions may encircle the affected joint (Fig. 5). Microscopically, the lesions display a micro-nodular growth pattern (Fig. 6). These nodules may coalesce into masses in advanced lesions. The lesions most often originate beneath the synovium, and the synovial lining is intact above the tumor (Fig. 7). In advanced lesions, these topographical relationships may be obscured by effacing mass formation. Cytologically, articular HS resemble HS in other sites – cytologically atypical, pleomorphic histiocytes predominate. However, a marked inflammatory infiltrate is usually recognizable within the lesions. Lymphocytes (mostly T cells), neutrophils and well-differentiated histiocytes are prevalent. Inflammation of the adjacent synovium with lymphocyte, plasma cells and histiocytes is also observed.
A history of anterior cruciate rupture or other traumatic injury to joints is associated with the development of articular HS. Synovitis has been reported in association with rupture of the cranial cruciate ligament. T cells and dendritic cells are major components of the inflammatory response. While no causal relationship necessarily exists between synovial inflammation and the development of HS, there is ample evidence of progression of inflammation to neoplasia in many inflammatory disease states (Nature. 2002; 420(6917):860–867)
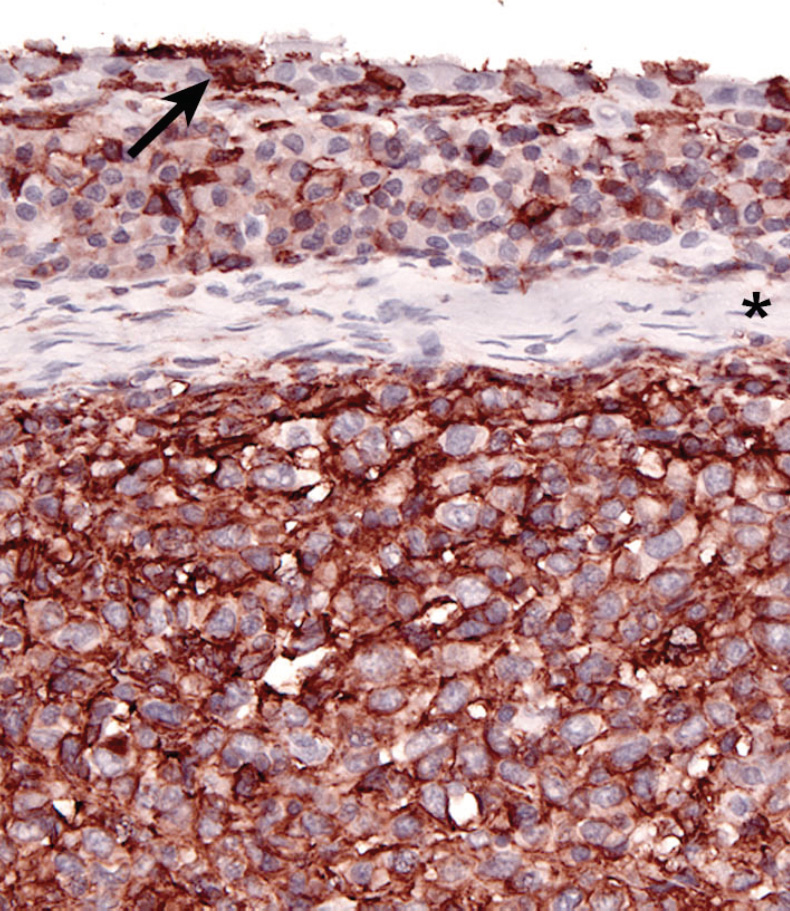
The immunophenotype of histiocytes in articular HS is identical to that of perivascular interstitial DCs (CD1a+ CD11c/CD18+) rather than synovial type A cells (CD1a- CD11b/CD18+), which are intercalated between synovial type B cells (synovial fibroblasts) in the synovial lining. Synovial type A cells don’texpress CD11c (Fairley RA, LeFranco L and Moore, PF - unpublished data). Synovial cell sarcoma (SCS) is a fibroblastic proliferation, which is likely derived from synovial type B cells (specialized synovial fibroblasts). Synovial fibroblasts are highly synthetic cells that can produce a diverse array of inflammatory mediators, including chemokines, which attract macrophages (Arthritis Res. 2000;2(5):356–360). Hence, it is not surprising that synovial cell sarcomas are infiltrated by large numbers of CD18+ histiocytes in most instances (Moore, PF - unpublished data). This may lead to confusion of SCS with articular HS. However, a dominant CD18- neoplastic population (interpreted as synoviocytes) is present among the many CD18+ histiocytes. This contrasts with the uninterrupted sheets of CD18+ cytologically atypical histiocytes observed in articular HS (Fig. 8).
-TOP-
Central Nervous System Histiocytic Sarcoma
The central nervous system may be infiltrated by HS as a primary location or as a consequence of metastatic spread of disseminated HS. Extracranial metastasis of primary CNS HS must be extremely rare, since it has yet to be reported. Histiocytic sarcoma of the CNS most often originates in the leptomeninges (Fig. 9), and the lesions are composed of large numbers of mixed inflammatory cells (lymphocytes, histiocytes and plasma cells) and cytologically atypical histiocytes (Fig. 10). The frequency of the latter cells is quite variable between cases. In the only case series of CNS HS published, Pembroke Welsh Corgis were overrepresented (7 of 15 dogs). This is not a breed that is otherwise commonly affected by HS. In reviewing the UC Davis data (> 70 dogs with primary CNS HS), Pembroke Welsh corgis were not represented. Recently this situation has changed and Welsh Corgis are now overrepresented in the UC Davis series (Dickinson, PJ, University of California, Davis – personal communication). The CNS lesions most commonly presented as focal, solitary subdural masses, and less commonly as diffuse meningeal infiltrates. The lesions appeared to arise in the leptomeninges and subsequently involved the brain. Metastasis beyond the CNS did not occur. Neoplastic histiocytes in solitary mass lesions expressed MHC class II molecules, Iba1 (ionized calcium binding adaptor molecule 1), and scavenger receptors expressed by macrophages (CD163 and CD204) (J Vet Diagn Invest. 2011;23(1):127–132). The macrophage scavenger receptors were not expressed in the diffuse meningeal HS. The cell and tissue distribution of the macrophage scavenger receptors (CD163 and CD204) have not been extensivley studied in canine tissues, so it is difficult to reconcile this data with our data, which indicate interstitial DC markers (CD1a, CD11c and MHC class II) are expressed by neoplastic histiocytes in CNS HS in our published report (Vet Pathol. 2002;39(1):74–83) and subsequent unpublished data. Macrophage scavenger receptors are present on subsets of interstitial DCs, so this could be one potential point of reconciliation of the divergent data. Also, dendritic cells are located in the meninges and choroid plexi, but not in the brain. Hence, the apparent site of initiation of CNS HS does have a resident population of interstitial DCs from which HS could develop.
-TOP-
Dendritic cell leukemia
Peripheral blood involvement in HS is rare. There have been two reports of DC leukemia in dogs, in both instances large numbers of atypical histiocytes were observed in peripheral blood (30,000 – 60,000 per microliter). In both dogs the DC lineage (CD1+ CD11c+ CD11d- MHCII+) was confirmed by extensive immunophenotyping of cell smears or by flow cytometry. Diffuse infiltration of affected organs (bone marrow, spleen, lung and liver) without mass formation was characteristic of a leukemic infiltrate. This is also the pattern of infiltration exhibited by hemophagocytic HS. However this latter disease does not involve peripheral blood and is associated with a responsive extravascular hemolytic anemia and marked erythrophagocytosis by neoplastic histiocytes, which express CD11d. CD11d was not expressed by neoplastic histiocytes in either dog.
-TOP-
